
Logout
If you want to log out click in LogOut

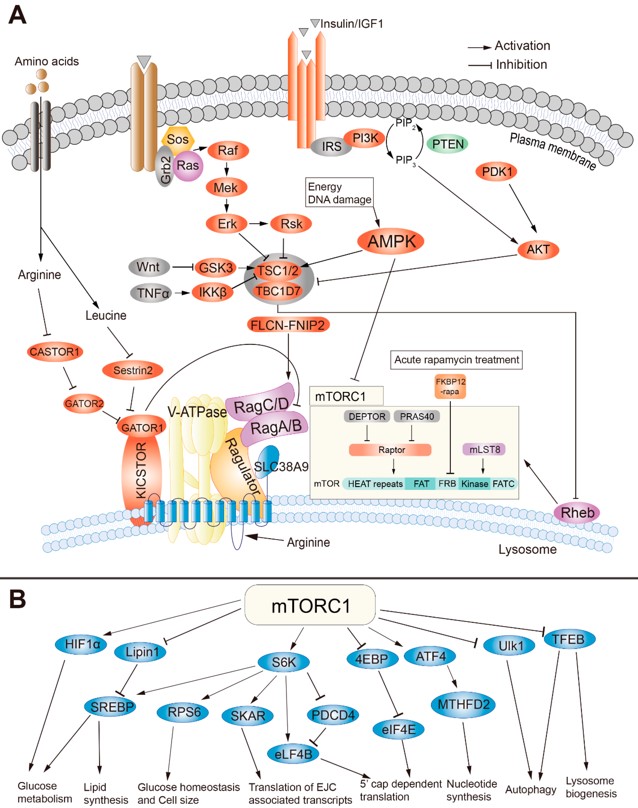
mTOR pathway (mamalien or mechanistic target of rapamycin mTOR), an atypical serine/threonine kinase, through two distinct protein complexes mTORC1 and mTORC2, coordinates eukaryotic cell growth and metabolism with environmental inputs including nutrients and growth factors. Other external stresses, such as oxygen deprivation (hypoxia), radiation, high salt concentration, DNA topoisomerase inhibitors, and histone deacetylase inhibitors, also regulate this pathway (1,6).
The distinct roles of mechanistic target of rapamycin were identified in gene transcription, protein synthesis, tissue regeneration and repair, oxidative stress, immunity, aging, and cell death that include autophagy and apoptosis, insulin resistance, adipogenesis, and T-lymphocyte activation (2,4).
You can custom your own SignArrays® with the genes of interest of your choice, according to your project, you just have to download and complete our Personalized SignArrays® information file and send it at contact@anygenes.com