
Logout
If you want to log out click in LogOut

Do you need to test your new therapeutic drugs on robust ex-vivo models via Histoculture Drug Response Assays ?
The relevance of some models can be sometimes questioned because the heterogeneity of tumors for example is not recreated. We have a solution!
Use our new ex-vivo histoculture protocols to explore directly on patient-derived models the effect of your new therapeutic drugs : the perfect model for clinical simulation studies.
Based on Histoculture Drug Response Assays (HDRA), this AnyGenes® service is perfect to:
- monitor drug response by identifying the best combination(s) of therapeutic molecules for each individual patient (personalized medicine)
- identify potential mechanism(s) of resistance
- attest to the clinical significance of your ex-vivo models
- discover molecular signature and biomarkers thanks to our quantitative real-time PCR SignArrays® system, specific to signaling pathways, directly on the sliced patient-derived biopsies. Indeed, sensitive, reliable and highly reproducible, qPCR arrays are the solution to explore gene expression between your different experimental conditions and understand all the drug induced effects on each patient-derived model.
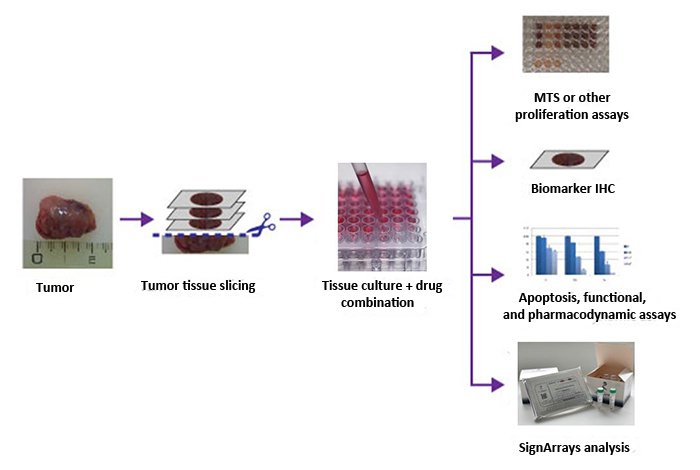
All Histoculture Drug Response Assays (HDRA) experiments and data analysis are performed on our high-throughput molecular platform to get you very robust results in a very short time! You just have to send us your biopsies according to our strict recommended conditions in order to guarantee the quality of each sample and experiment.
Bibliography:
Yoon YS et al. Development and Applicability of Integrative Tumor Response Assays for Metastatic Colorectal Cancer. Anticancer Res. 2017 Mar;37(3):1297-1303.
Delyon et al. Validation of a preclinical model for assessment of drug efficacy in melanoma. Oncotarget. 2016 Mar 15; 7(11): 13069–13081.
Jung PS et al. Progression-free survival is accurately predicted in patients treated with chemotherapy for epithelial ovarian cancer by the histoculture drug response assay in a prospective correlative clinical trial at a single institution. Anticancer Res. 2013 Mar;33(3):1029-1034.