
Logout
If you want to log out click in LogOut

Cellular senescence, including replicative senescence (RS) and stress-induced premature senescence (SIPS), constitute a permanent state of cell cycle arrest that occurs in proliferating cells subjected to different stresses.
This irreversible proliferative arrest process is associated with changes in chromatin organization, gene transcription, and protein secretion either in vitro or in vivo (1,2,3). The senescent cells are characterized by: prolonged cell cycle arrest, transcriptional changes, acquisition of a bioactive secretome, known as the senescence-associated secretory phenotype (SASP), macromolecular damage, and deregulated metabolism (4).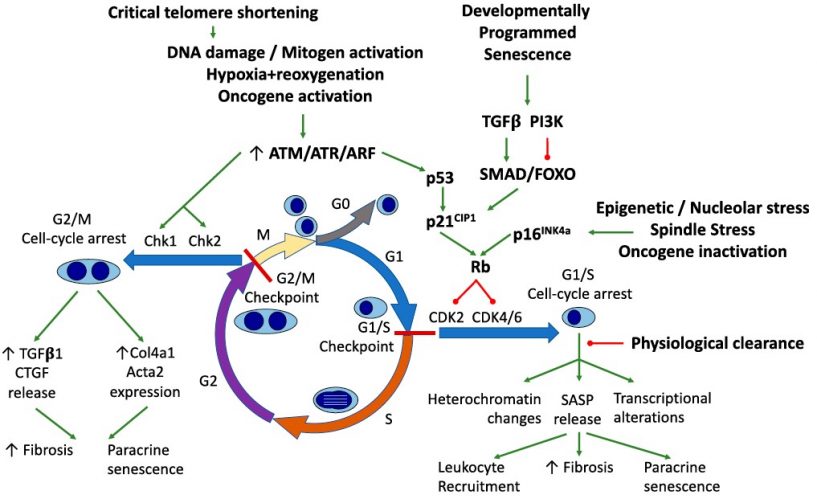
Senescence is a cellular defense mechanism that prevents the cells to acquire an unnecessary damage. The senescent state is accompanied by a failure to re-enter the cell cycle in response to mitogenic stimuli, an enhanced secretory phenotype and resistance to cell death (1).
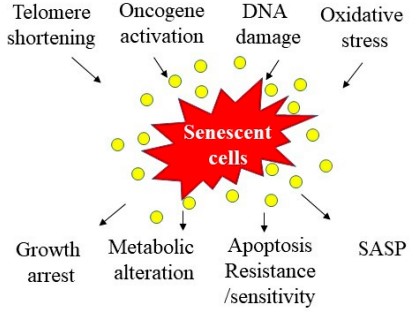
Due to the multiplicity of stress signals and effector pathways involved in its execution, senescence is associated with various phenotypes the characteristics of which depend on the signals that induced senescence, the originating cell type, the time elapsed since senescence initiation, and the site where senescent cells are located(4).
Senescence is one of the causative processes of various pathologies like kidney disease, aging and aging-related disorders including Alzheimer’s disease, diabetes and associated diabetic complications, cardiovascular disease, fatty liver disease (1,2,3,5).
Besides its pathological role, senescent cells can also play a positive role. In embryogenesis and tissue remodeling, senescent cells are necessary for the proper development of the embryo and tissue repair. In cancer, senescence acts as a powerful barrier to prevent tumorigenesis (1).You can custom your own SignArrays® with the genes of interest of your choice, according to your project, you just have to download and complete our Personalized SignArrays® information file and send it at contact@anygenes.com