
Logout
If you want to log out click in LogOut

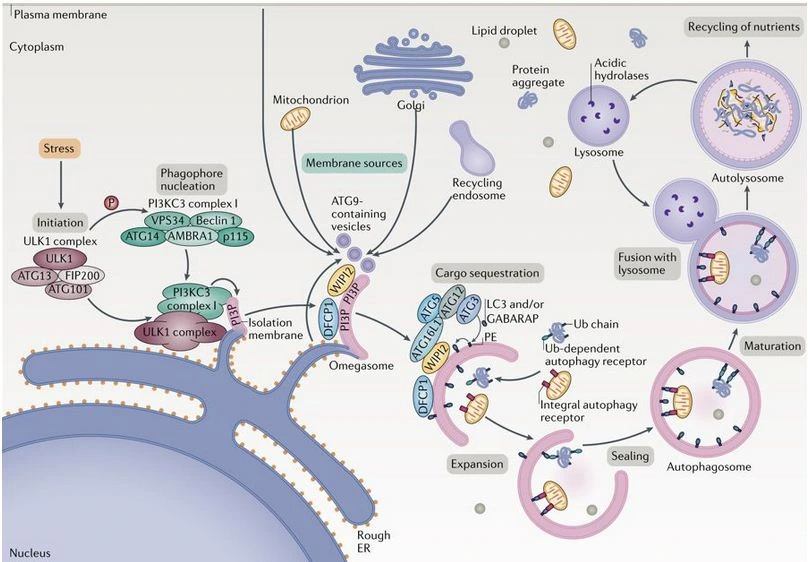
Autophagy pathway is an adaptive process in response to cellular stress as nutrient starvation, oxidative stress, hypoxia, protein aggregation... It prevents cell damage and promote cell survival. It is a crucial molecular pathway for the preservation of cellular and organismal homeostasis (1,2).
During this process, key autophagic factors (ATG) orchestrate the formation of a double-membrane vesicle, known as the autophagosome. This encapsulates cellular components and fuses with lysosomes, resulting in the degradation of its contents through the activities of lysosomal hydrolases. The control of these factors is very important to ensure cellular homeostasis (3).
In eukaryotes, there are two conserved autophagy pathways, macro-autophagy and micro-autophagy.This leads to the formation of a double-membraned structure known as the autophagosome. This autophagosome encapsulates a portion of the cytoplasm. Subsequently, the autophagosome merges with lysosomes (or vacuoles in yeasts and plants), which break down the contents.
The ULK complex is the central regulator in the beginning of macro-autophagy. It contains the scaffold protein FIP200 (also known as RB1CC1), ATG13, ATG101 and the serine/threonine kinases ULK1 or ULK2. Mainly, mechanistic target of rapamycin complex 1 (mTORC1) inhibits its activity.
Endosomal micro-autophagy, a form of micro-autophagy in mammalian cells, involves the formation of multivesicular bodies within endosomes.
The endosomal micro-autophagy occurs naturally. It also triggers during early periods of amino acid starvation. This process helps break down certain proteins found in the cytoplasm. Specifically, adaptors involved in macro-autophagy, like SQSTM1, NDP52, NBR1, TAX1BP1, and NCOA4, which is responsible for ferritin (9).
A recent study on replicative crisis suggests that autophagy is an integral component of the tumor suppressive crisis mechanism. The cancer onset requires a loss of autophagy function.
During the crisis, on the one hand, proteins linked to autophagy increases. This includes lysosomal-associated membrane protein 1 (LAMP1) and ATG5–ATG12 conjugate (for phagophore biogenesis). In addition to the lipidated form of microtubule-associated protein 1A/1B-light chain 3 (LC3), LC3-II (for phagophore expansion). On the other hand, the autophagy target polyubiquitin-binding protein P62 (also known as SQSTM1) decreases (7).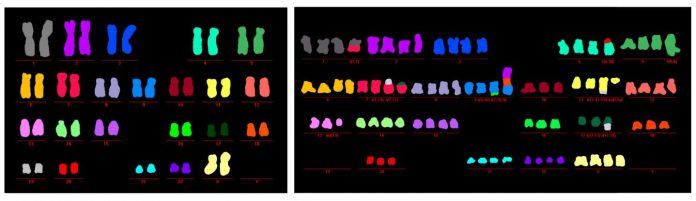
Left: The 23 pairs of chromosomes of cells in which autophagy is functioning look normal and healthy with no structural or numerical aberrations (each color represents a unique chromosome pair).
You can custom your own SignArrays® with the genes of interest of your choice, according to your project, you just have to download and complete our Personalized SignArrays® information file and send it at contact@anygenes.com