
Logout
If you want to log out click in LogOut

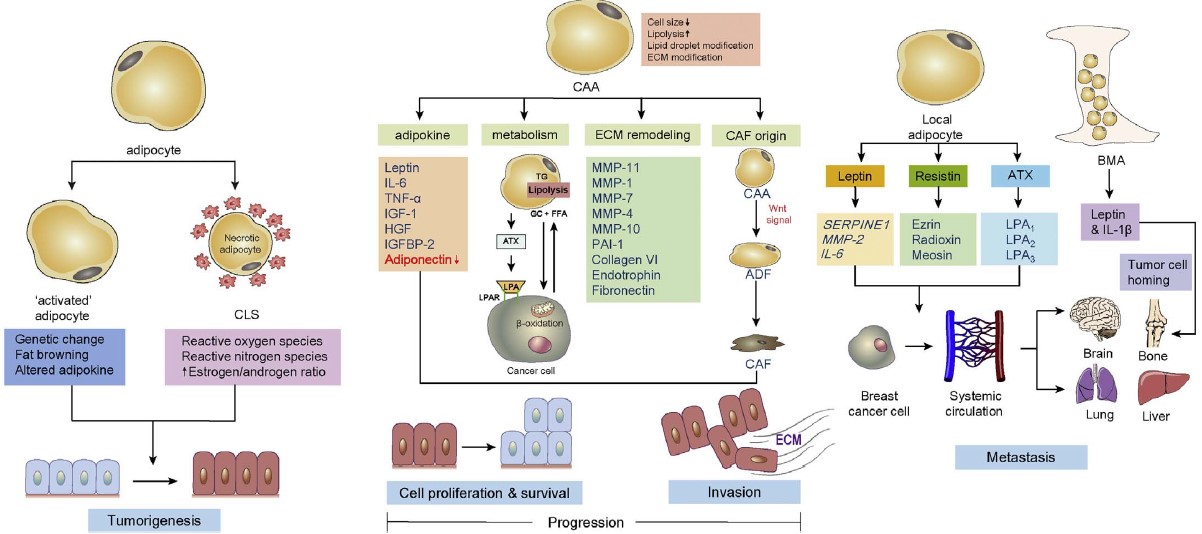
In mammalian cells, the transcription factors associated with adipogenesis control the differentiation of adipocytes. This includes the CCAAT/enhancer binding proteins (C/EBPs)(C/EBPα, β, and δ) and peroxisome proliferator-activated receptor γ (PPARγ). Fatty acid synthase (FAS), adiponectin, and fatty acid binding protein 4 (FABP4) combine to form mature adipocytes.
In addition to PPARγ and C/EBPα, other transcription factors positively regulate adipocyte differentiation. The Kruppel-like factors (KLFs) are among them. Activation of the KLF transcription factors KLF4, KLF5, KLF9, and KLF15 accompanies adipocyte differentiation in 3T3-L1 cell lines.
Researchers have shown that ectopic expression of KLF15 in NIH 3T3 cells induces lipid accumulation and expression of PPARγ. This suggests that KLF15 plays an important role in adipogenesis.
Expression of the active form of CREB in 3T3-L1 pre-adipocytes is sufficient to induce adipogenesis. CREB induces accumulation of Triglyceride and expression of two adipocyte marker genes, PPARγ and fatty acid binding protein.
In vitro studies show that transforming growth factor β (TGF-β) target the transcription factors linked to adipogenesis. PPAR, C/EBPβ, and C/EBPδ factors, followed by TGF-β-mediated adipogenesis inhibition. TGF-β inhibits adipocyte differentiation by interacting with C/EBP and repressing its transcriptional activity (1,4).
You can custom your own SignArrays® with the genes of interest of your choice, according to your project, you just have to download and complete our Personalized SignArrays® information file and send it at contact@anygenes.com