
Logout
If you want to log out click in LogOut

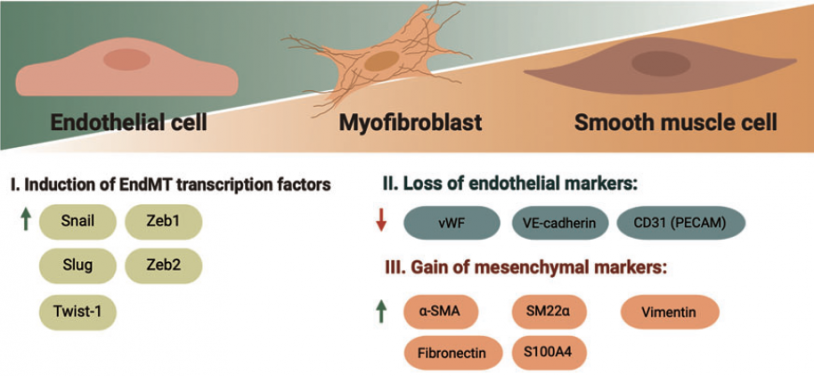
The EndMT process involves significant changes in the endothelial cell phenotype, both in terms of appearance and function. These changes are evident at the morphological, functional, and molecular levels.
Morphologically, the cells undergo alterations in cell polarity and undergo a remarkable restructuring of the cytoskeleton. At the molecular level, the endothelial cells start expressing genes specific to mesenchymal cells and produce corresponding proteins. This includes α-smooth muscle actin (α-SMA), extra domain A (EDA) fibronectin, N-cadherin, vimentin, fibroblast-specific protein-1 (FSP-1), fibroblast activating protein (FAP), and fibrillar collagens type I and type III.Concurrently, there is a gradual decrease and eventual loss of endothelial cell-specific proteins. Among them von Willebrand factor (vWF), CD31/platelet-endothelial cell adhesion molecule-1 (CD31/PECAM-1), and vascular-endothelial cadherin (VE-cadherin) (3).
EndMT can occur under pathological conditions such as inflammation, oxidative stress, high blood sugar, and low sheer stress. These stimuli activate downstream signaling pathways to induce EndMT. Many researchers have widely reviewed many relevant signaling pathways. The transforming growth factor-beta (TGF-β) signaling pathway considered the most important influencer of EndMT. Particularly the canonical downstream SMAD pathway (4).
You can check the biomarker list included in this pathway, see below: