Logout
If you want to log out click in LogOut

In transplantation, the antibody mediated rejection (ABMR) is currently a major thread for the long term graft survival. However the diagnosis of the ABMR remains still difficult and according to the last Banff classification the diagnosis of ABMR requires the evidence of current/recent antibody interaction with graft vascular endothelial cells: including one of following: linear c4d (a fragment of complement) staining in peritubular capillaries; or moderate microvascular inflammation; or increased endothelial injury related gene expression with thoroughly bio-statistically validated result. In the absence of easy access to transcriptomic analysis of graft samples, relying only on C4d staining or microvascular inflammation is clinically unsatisfactory.
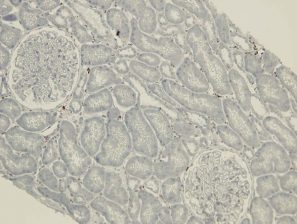
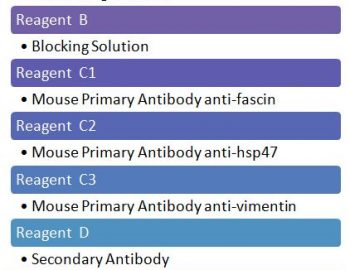
The ligation of HLA molecules on the vascular endothelial cells with corresponding antibodies and/or activation of complement following Antigen-Antibody complex formation can trigger cell stress response and by various signaling pathways it can affect the endothelial transcriptional profile and induce an up-regulation of molecules involved in inflammation, coagulation, cell motility and endothelial repair, a process reminiscing Endothelial to mesenchymal transition (EndMT). This EndMT process now can be detected on paraffin tissue by expression of fascin, hsp47 and vimentin (figures). The expression of these EndMT markers on the renal grafts is associated with the presence of DSA and predicts poor graft outcome.
fascin
hsp47
vimentin
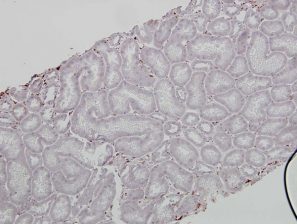
No peri-tubular capillary EndMT marker staining in Normal renal grafts:
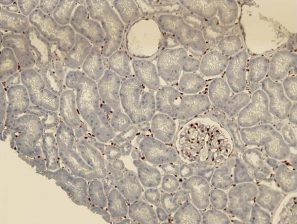
Peri-tubular capillary EndMT marker staining in renal grafts with antibody mediated rejection:
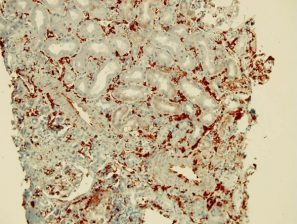
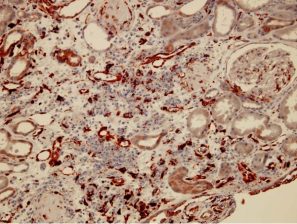
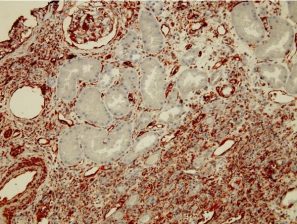
The EndMT Test, detects the endothelial cell injury and its implication in the antibody mediated rejection (see description above)
For more technical information, click here
EndMT Test prices
| Reference type : EDMT-20 | Perfect Master Mix SYBRG® |
| Number of tests | Price before TAX |
|---|---|
| 20 | * |
Efficient tools to novel targeted therapies offer promise in improving patient outcomes, notably in the context of cancer. However, it is crucial to identify which patients will benefit from targeted therapy. Biomarkers to predict or monitor therapy response are becoming essential.
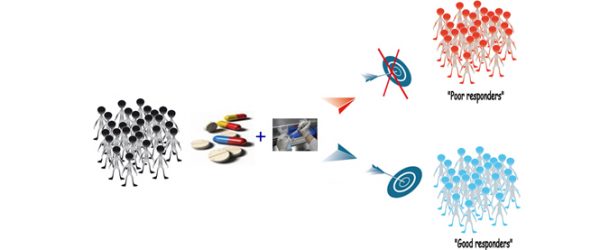
Predictive biomarkers offer significant benefit by refining the treated population to a subgroup that has a greater prospect of benefit from therapy. This provides efficacy benefits, but also minimizes the overall toxicity and cost of treatment. This new concept is the Personalized Medicine.
Personalized Medicine aims to achieve optimum medical outcomes in the management of patient's diseases by using molecular analysis. In this quest, AnyGenes® developed a novel approach based on combined biomarker's pathways analysis thanks to it own SignArrays® system. Many targeted therapies are being studied in clinical trials, and many more are in preclinical testing (research studies in vitro or with animals).
To accelerate and improve Targeted Therapies development, AnyGenes® can provide qPCR arrays-focused pathways (SignArrays®), built specifically for each targeted therapy. Therefore, AnyGenes® can also help to identify the most efficient biomarkers combination with analytically and clinically validated assays.
AnyGenes® provides clinicians with validated biomarkers assays to monitor targeted therapies response.